DNA Damage in Atrial Fibrillation: a Promising Treatment Target?
Atrial Fibrillation (AF) is the most common cardiac arrhythmia affecting 2-3% of the population in Western countries. AF causes irregular and often rapid heartbeat, as well as an increased risk of stroke, heart failure and mortality.
Initially, patients diagnosed with AF experience self-terminating episodes, however, given that AF is a progressive disease, those episodes reoccur with increasing frequency and duration.
Unfortunately, AF treatments are not always effective, resulting in a percentage of recurrence and side effects. The difficulty of treatment predicts a great need to dissect root causes of AF with an ultimate goal to develop drug therapies focused on its pathological mechanisms.
Atrial fibrillation causes DNA damage
Interestingly, new findings reveal induction of DNA damage as a mechanistic root cause of AF, which subsequently recruits DNA repair machineries, such as the protein poly-ADP-ribose polymerase (PARP1).
In order to repair damaged DNA molecules, PARP1 consumes nicotinamide adenine dinucleotide (NAD+). NAD+ is a cofactor that is crucial to the energy metabolism of cells, including cardiomyocytes; the contracting cells from the heart.
Therefore, a decrease in NAD+ levels induces a scenario of energy failure which promotes electrical and contractile dysfunction in cardiomyocytes. This newly elucidated role of DNA damage in AF opens opportunities for novel treatment strategies.
Recent studies have shown positive results when administering PARP inhibitors or NAD+ replenishment drugs.
These two drug categories seem to promote beneficial effects in experimental AF, as well as in coronary artery disease (myocardial infarction) and lamin A/C mutant induced dilated cardiomyopathy and peripartum-cardiomyopathy.
In the review article entitled “DNA Damage, an Innocent Bystander in Atrial Fibrillation and Other Cardiovascular Diseases?”, we describe the role of DNA damage and its influence on cellular energy metabolism in AF and other cardiovascular diseases and describe why these diseases often act together.
We also discuss potential pharmacological targets for AF and highlight future directions for clinical trials using drugs directed to the PARP1-NAD+ pathway with the objective to preserve quality of life and to attenuate severe complications such as heart failure or stroke in patients with AF.
Read more about this review article via Frontiers website (https://doi.org/10.3389/fcvm.2020.00067).
This article is part of the special issue ‘Innovative approaches to tackle atrial fibrillation’
The authors thank Miles Henderson (https://www.linkedin.com/in/miles-henderson/) for graphic design of the figures.
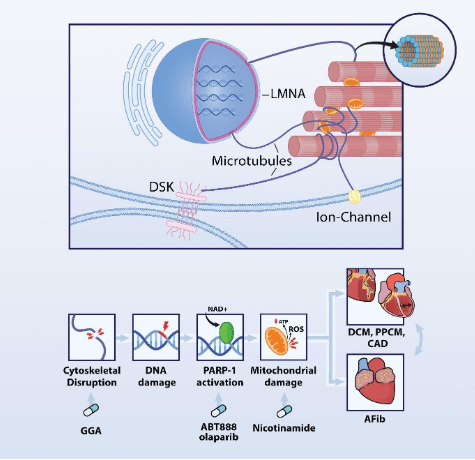
Figure 1: DNA damage-PARP1-NAD+ axis is involved in AF and additional cardiovascular diseases. AF causes DNA damage, PARP1 activation and excessive depletion of the NAD+ levels resulting cellular energy failure. Key modulators within this axis are druggable targets PARP1 inhibitors and NAD+ replenishment, protecting against atrial fibrillation (Afib), coronary artery disease (CAD), dilated cardiomyopathy (DCM), and peripartum cardiomyopathy (PPCM).
Do you know someone who might be interested in this?