Cell-free circulating mitochondrial DNA: a potential blood-based marker for atrial fibrillation
Atrial fibrillation (AF) is the most common progressive cardiac tachyarrhythmia globally. AF is present in 3% of the total population, with a higher prevalence in the elderly, and is associated with serious complications such as stroke, heart failure, impaired cognitive function, and increased mortality. Early recognition of AF is essential to prevent disease progression from recurrent intermitted episodes to finally the permanent AF stage. This progression is accompanied by a gradual increase in therapy failure and the end-stage can, even with extensive therapy, not be treated.
At present, AF can only be diagnosed with a surface electrocardiogram when a patient already suffers from AF. In addition, this rhythm registration cannot assess the stage of AF, which is essential for selection of the appropriate therapy. Hence, early recognition of AF and the start of effective treatment is seriously hampered. Therefore, there is an urgent need to develop successful diagnostic biomarkers for AF.
Blood-based Mitochondrial DNA as marker to stage atrial fibrillation
The use of serum biomarkers is an interesting approach to assess (patho)physiological processes and therapeutic responses. Serum biomarkers are used in the identification and management of several diseases, including heart failure, myocardial infarction, cancer, and diabetes. So far, no specific serum biomarkers to assess AF stage and to predict the outcome of AF treatment are available. Nevertheless, several AF serum biomarkers, such as troponin (myocardial injury), brain natriuretic peptide (cardiovascular stress), creatinine (renal dysfunction), C-reactive protein (inflammation), and fibroblast growth factor 23, have been associated with AF pathology. Only in 2016, the European Society of Cardiology implemented the use of troponin and natriuretic peptide into their guidelines, to predict stroke and bleeding risk in AF patients.
Cell-free circulating mitochondrial DNA (cfc-mtDNA) is successfully used as a biomarker for conditions associated with mitochondrial dysfunction or stress, such as cancer progression, cardiac arrest survival, and diabetes mellitus. Cfc-mtDNA acts as a damage-associated pattern (DAMP), causing inflammation, which might be associated with the inflammation seen in AF patients and an experimental mouse model for AF. We recently observed mitochondrial dysfunction to underlie experimental and human AF (Wiersma Cells 2019). As mitochondrial dysfunction may initiate the release of cfc-mtDNA in the circulation, we sought to evaluate the value of cfc-mtDNA as a blood-based marker for AF stage, sex differences, and AF recurrence after treatment, in a population of patients undergoing AF treatment (electrical cardioversion (ECV) or pulmonary vein isolation (PVI)) or cardiac surgery.
mtDNA levels associate with paroxysmal AF: especially in male patients
In our study, Dr. Marit Wiersma from the team of Prof. Bianca Brundel (Amsterdam UMC, VUmc) measured the levels of two mtDNA genes, COX3 and ND1, in 84 control patients (C), 59 patients undergoing cardiac surgery without a history of AF (SR), 100 paroxysmal (PAF), 116 persistent (PeAF), and 20 longstanding-persistent (LS-PeAF) AF patients undergoing either cardiac surgery or AF treatment (electrical cardioversion or pulmonary vein isolation) at the Erasmus Medical Center Rotterdam.
Interestingly, cfc-mtDNA levels were significantly increased in PAF patients undergoing AF treatment, especially in males and patients with AF recurrence after AF treatment (Figure 1). In PeAF and LS-PeAF, cfc-mtDNA levels gradually decreased. Importantly, cfc-mtDNA in serum may originate from cardiomyocytes, as in vitro tachypaced cardiomyocytes release mtDNA in the medium.
The findings suggest that cfc-mtDNA is associated with AF stage, especially in males, and with patients at risk for AF recurrence after treatment.
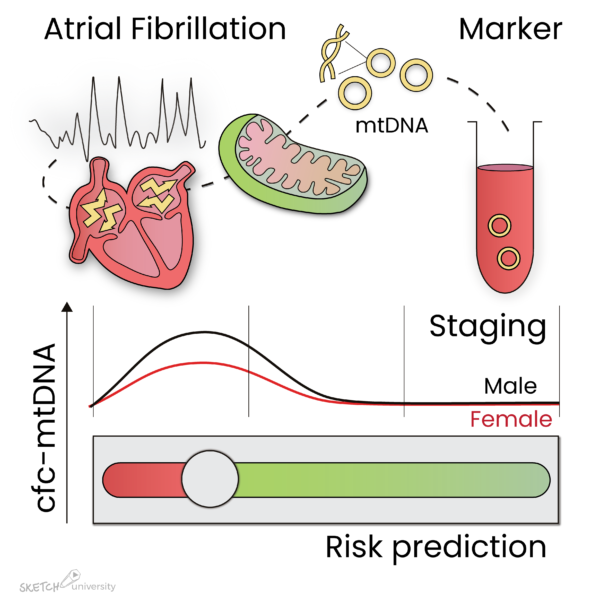
Figure 1: Atrial fibrillation (AF) causes damage to the mitochondria in the atria, which result in the release of mtDNA in the circulation. During paroxysmal AF, mtDNA levels are increased, especially in male patients, and reduce again during progression of the arrhythmia. In addition, mtDNA levels are elevated in patients who reveal recurrence of AF after ablative therapy. Therefore, mtDNA levels may represent a marker to stage AF and predict AF recurrence after treatment.
AFIP congratulates Dr. Marit Wiersma, Prof. Dr. Bianca Brundel and Prof. Dr. Natasja de Groot with publication of their findings in the scientific journal Cells 2020, 9(5), 1159; https://doi.org/10.3390/cells9051159
Furthermore, we thank Sketch University for design of the graphic abstract.
Do you know someone who might be interested in this?






